Tuberculosis Care and Prevention

Tuberculosis Care and Prevention

From Directly Observed Treatment to Digital Adherence Technology (DOT to DAT)
In collaboration with the Uganda TB Implementation Research Consortium, DOT to DAT is an implementation science study testing the use of Digital Adherence Technology in improving adherence to tuberculosis medication. We have enrolled 18 trial sites that meet eligibility criteria and randomized these sites into the study months when they will switch to the intervention. We have also begun the process of introducing 99DOTS at sites and training health facility staff to use the technology.
People served
People with tuberculosis
Funder
TB REACH

Trace Ultra Result iNsight in TB Screening (TURN-TB)
TURN-TB (Trace Ultra Result iNsight in TB screening) is a case-control study that aims to compare baseline Xpert® MTB/RIF Ultra (“Ultra”) assay “trace-positive” individuals to positive and negative controls and includes a longitudinal cohort study with TB-negative controls. Partly in coordination with the STOMP-TB study, the study conducts community-based TB screening as well as recruitment from local facilities, and enrolls participants with trace-positive results for an extensive baseline clinical and laboratory evaluation.
People served
People at risk of a tuberculosis infection
Funder

Clinic versus Hotspot Active Case Finding/TPT Strategy Evaluation for Tuberculosis (CHASE-TB)
Key technological innovations have converged to make community-based campaigns to provide Active Case Finding (ACF) and TB Preventive Treatment (TPT) increasingly feasible over the past 5 years:
1. Novel mobile x-ray with artificial intelligence = human, can screen masses.
2. GeneXpert with results in ~2 hours, more accurate than microscopy, faster than culture
3. Novel regimens with shortened duration; low drug burden and cost
4. Venue based screening (VBS), future active case finding
Therefore, the overall goal of the study is to evaluate the comparative effectiveness and implementation of this combination intervention under two different approaches; a clinic-based strategy and a community “hotspot-focused” approach.
People served
Adults 15 years and above, plus their TB contacts 5 years and above
Funder
United States National Institutes of Health (NIH)-funded cluster randomized trial (5R01HL138728-06; PIs: Kendall, Dowdy, Katamba)
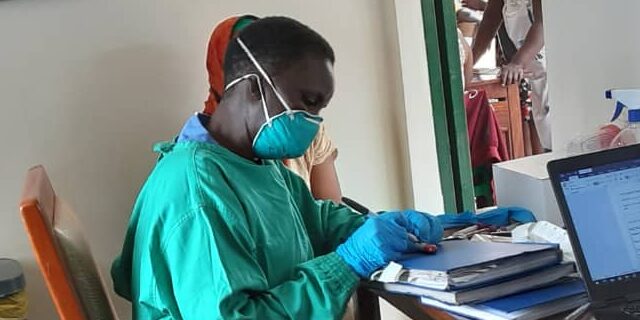
Evaluation of rapid non-sputum based biomarker-based tests for tuberculosis diagnosis
The study seeks to develop and evaluate non-sputum based tests for tuberculosis (TB), which is a leading priority for TB diagnostics research. Currently, we are focused on ways of processing tongue swabs for molecular detection of TB among non-hospitalized participants (age ≥12 years). Prior to sputum collection, participants provide tongue swabs for diagnostic accuracy testing in reference to routine sputum-based TB results. Tongue swab testing is expected to facilitate more people in the community to get tested (and treated) since tongue swab collection is simple, safe and noninvasive.
People served
Outpatients 12 years and above with suspected pulmonary Tuberculosis (TB)
Funder
Global Health Labs

Evaluating the Impact of Cash Transfers on Tuberculosis (TB) Care - (ExaCT TB study)
TB extremely affects the poorest and most vulnerable populations. Unfortunately, a number of patients who seek TB care do not complete diagnostic evaluation and the principal reason is the failure to address their social and economic barriers. Notably, patients who develop TB spend >20% household income(catastrophic costs) just to obtain a diagnosis because of the high direct and opportunity costs related to accessing TB diagnostic services. This significant financial burden makes them less likely to complete testing and initiate treatment, leading to increased transmission, morbidity and mortality. For this reason therefore, feasible and scalable social protection interventions that address known barriers to completion of TB diagnostic evaluation are urgently needed.
The ExaCT TB study aims to evaluate the effectiveness of a social protection intervention incorporating cash transfers and social support for patients suspected of having TB in Uganda.
All eligible patients undergoing clinical evaluation for pulmonary TB at 10 community health centers receive a Cash Transfer (CT) of UGX 20,000 at the time of sputum submission via a local mobile money service provider approved by the Uganda Communications Committee.
CTs may facilitate completion of TB diagnostic evaluation by mitigating common financial barriers among patients accessing TB care in Uganda.
People served
Adults(aged 18years and above) seeking TB diagnostic evaluation at ten community health centers
Funder
Swedish Research Council

Re-Imagining TB Care in Uganda
The RTC initiative was originally conceptualized at the first TB Innovation Summit, which was co-organized by the Stop TB Partnership, Johnson & Johnson, United Nations Foundation, Global Fund to Fight AIDS, Tuberculosis and Malaria (“Global Fund”), and the World Economic Forum, in advance of the first United Nations High-Level Meeting (UNHLM) on tuberculosis (TB)in September 2018.
A broad spectrum of in-country and global stakeholders and partners, including Ministries of Health, country programmes, care providers (i.e., public and private, community health workers,etc.), and TB affected people and communities, including TB survivors, came together to discuss the need to modernize our thinking and approach in terms of how healthcare services are accessed and delivered in TB affected countries and the critical role that innovations, particularly digital health technologies can play to catalyze integrated, differentiated, and people-centered care and bring services closer to where the TB affected people and communities are taking into consideration their behaviors, daily routines (i.e., live, work, etc.), and preferences.
Uganda is one of the 30 World Health Organization-designated countries with a high burden of TB and TB/HIV. In 2020, it is estimated that 90,000 people developed TB, of which 29,113
(32%) were missed giving a TB treatment coverage of 68%. Uganda is one of the countries
where COVID-19 pandemic disrupted TB services. It is one of the 16 countries that accounted
for 93% of the drop in global TB notifications between 2019 and 2020 [WHO database].
People served
TB-affected people; Civil Society Organizations; Community Health Workers & Ministry of Health
Funder
KOICA and Stop TB Partnership

Rapid Research in Diagnostics Development for TB Network (R2D2 TB Network) Study
The R2D2 study aims to reduce the burden of TB worldwide through more accurate, faster, simpler, and less expensive diagnosis of TB Every year, more than 3 million people with TB remain undiagnosed and 1 million die. Better diagnostics are essential to reducing the enormous burden of TB worldwide.
The Rapid Research in Diagnostics Development for TB Network (R2D2 TB Network) brings together experts in TB care, technology assessment, diagnostics development, laboratory medicine, epidemiology, health economics and mathematical modeling with highly experienced clinical study sites in 10 countries.
Together, we will move the field forward by providing a transparent and partner-engaged process for the identification, evaluation and advancement of the most promising TB diagnostics.
People served
Adults with presumptive TB Children with presumptive TB People living with HIV
Funder
Global Health Labs, NorthWestern University, and U.S. National Institutes of Health
